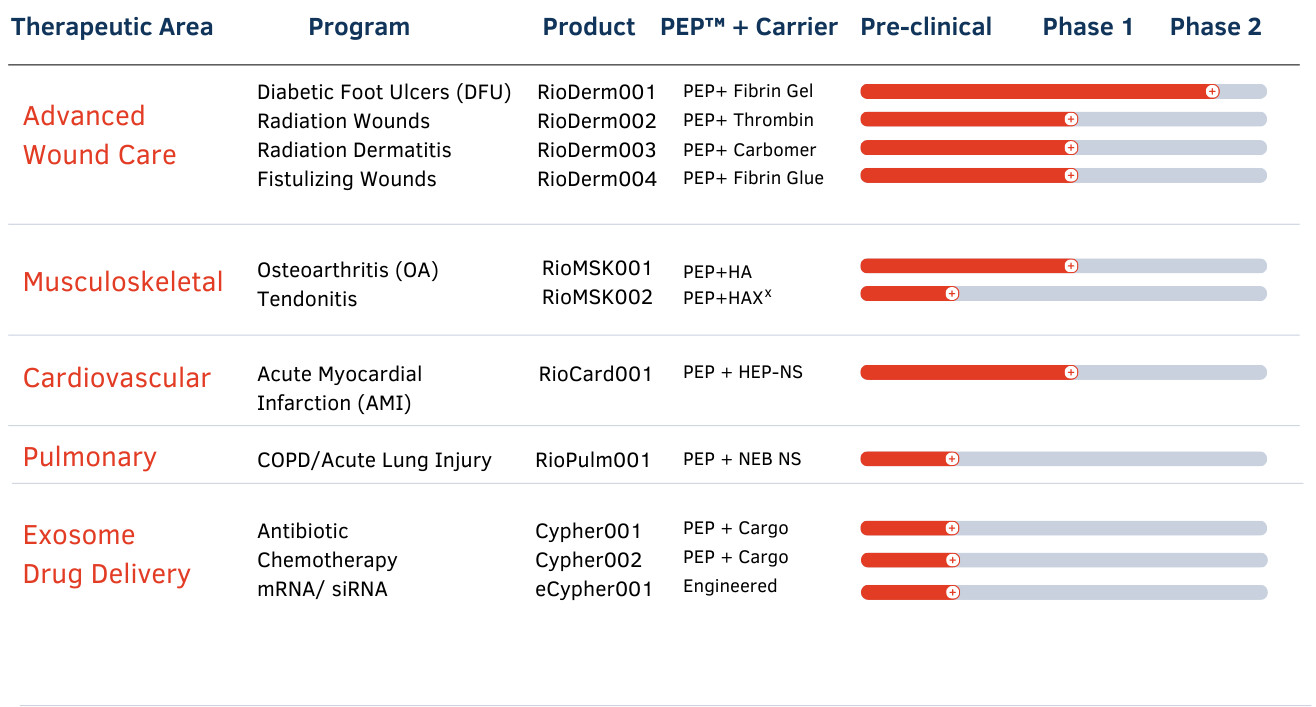
Our Pipeline
Our Pipeline
The Future of Medicine is Here:
Revolutionizing Exosome Science to Heal from Within!
At RION, we’re harnessing the power of platelet-derived exosomes, nature’s own delivery vehicles, to create the most advanced exosome clinical pipeline with PEP™
The Future of Medicine is Here:
Revolutionizing Exosome Science to Heal from Within!
At RION, we’re harnessing the power of platelet-derived exosomes, nature’s own delivery vehicles, to create the most advanced exosome clinical pipeline with PEP™